Building Organization Wide Approval Workflows
Organization-Wide Approval workflows are available for SDS and SDS/Chemical Management accounts only.
If your organization has a company-wide approval process for any products you bring on-site, you can mimic this process in your Chemical Management account using an Organization-Wide Approval workflow. While setting up an approval workflow is not required, using one is key if you need to keep detailed approval records for new SDSs added to your eBinder and you have a series of questions or forms you need completed as part of that approval process.
For more information on Organization-Wide Approval workflows, see Chemical Approval Tools.
Setting Up Organization-Wide Approvals
To set up an Organization-Wide Approval workflow for your account, select Settings and then click on Queue and Approval.
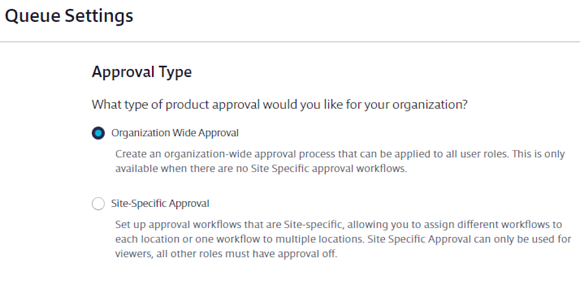
Select the radio button for Organization-Wide Approval. Click Save if necessary.
Note:
You may not be able to adjust settings on the Queue and Approval page if you have safety data sheets in your Queue.
Click Edit in the Approval Workflow section.
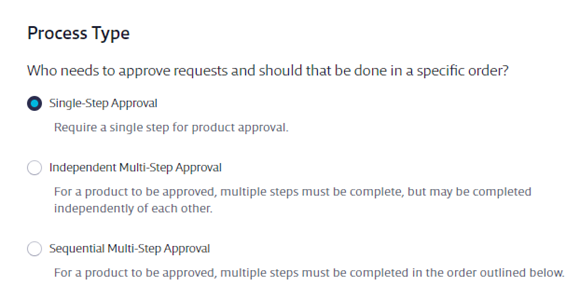
The first thing you need to select is the workflow Process Type. You have three options:
- Single-Step Approval allows any Administrator to approve a document in one step.
- Independent Multi-Step Approval allows the Account Administrator to set up a chemical approval process whereby several departments can have a say in whether a chemical should be approved for the organization or not. These steps can be completed in any order.
- Sequential Multi-Step Approval allows the Account Administrator to set up a chemical approval process whereby several departments can have a say in whether a chemical should be approved for the organization or not. These steps must be completed in order.
Creating a Single-Step Approval Workflow
A Single-Step Approval workflow allows any Administrator to approve a document in one step. Select Single-Step Approval in the Process Type section.
Under Workflow Approvers, select a single approver or multiple approvers that can take action on the safety data sheet on a first-come, first-served basis. You can select All Administrators, or a specific individual or individuals. Note that only Administrators or higher can be approvers for an Organization-Wide Approval workflow. If you need to include Managers as approvers, see Creating a Site-Specific Approval Workflow.
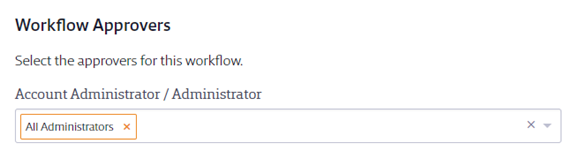
Under Submitter Instructions, enter any instructions you need the person who is asking to add the safety data sheet to read. If you have staff using your Chemical Management system in languages other than English, VelocityEHS recommends you use the Translate feature to ensure they see the correct wording. Click on Translate and then use Edit to enter the correct wording for the languages you need.
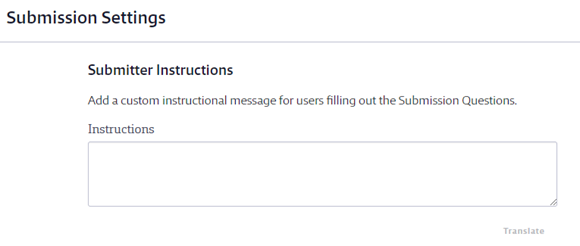
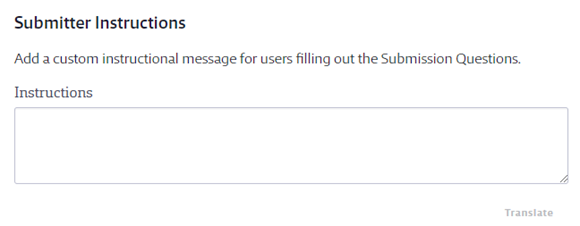
If you have staff that do not have logins to your Chemical Management account but who need to be notified when a safety data sheet is approved or not approved, you can enter their emails in the Additional Notification Email section. You can enter multiple emails as long as they are separated by commas. The system will send the Product Approval Update Email to these addresses.
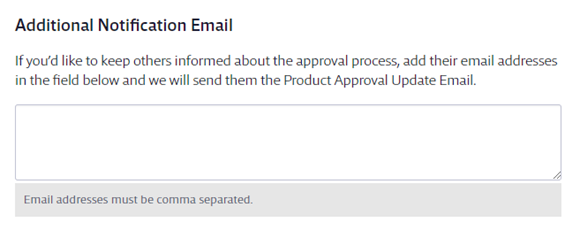
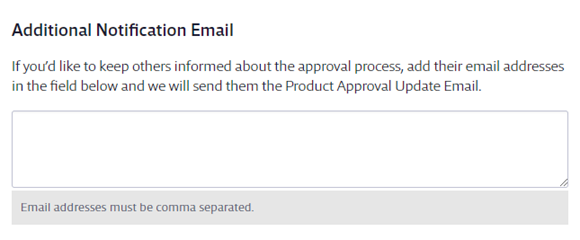
Use Attached Files if you have additional documents that need to be completed or reviewed by the submitter as part of the approval process. The submitter and any subsequent approvers can download the attached files and review them when submitting or approving the safety data sheet.
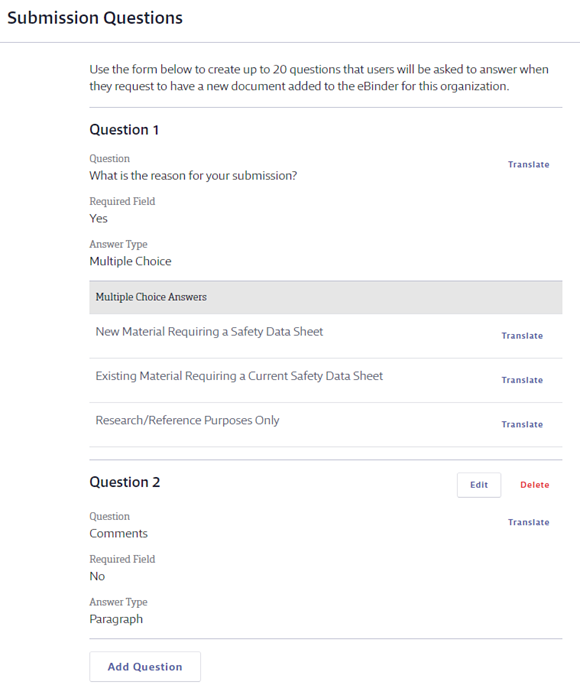
In Submission Questions create the questions you want the submitter to answer as part of asking to add the safety data sheet to your account. The Submission Questions are displayed to users whose safety data sheets require approval. Users that are Subject to Approval are directed to answer these questions after selecting a safety data sheet to add to the eBinder. See What does Subject to Approval mean? for more information.
Question 1 is always What is the reason for your submission? and cannot be edited or deleted. You can edit Question 2 by clicking on the Edit button. Question 2 can also be deleted.
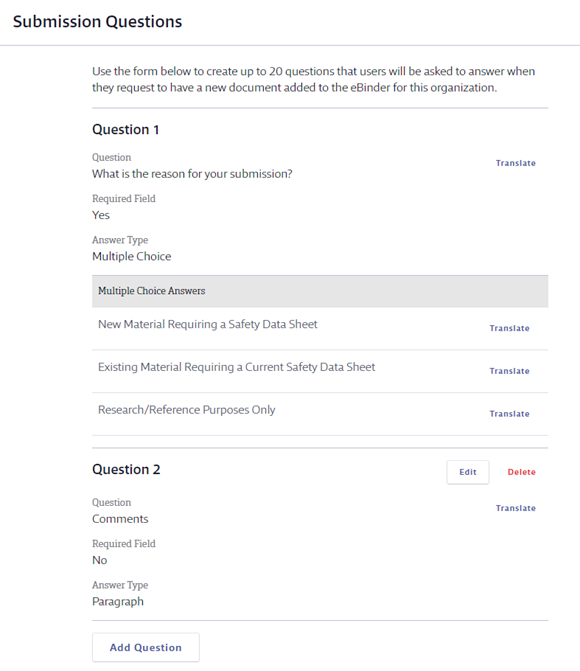
To add additional questions, click Add Question. You can have a total of 20 questions.
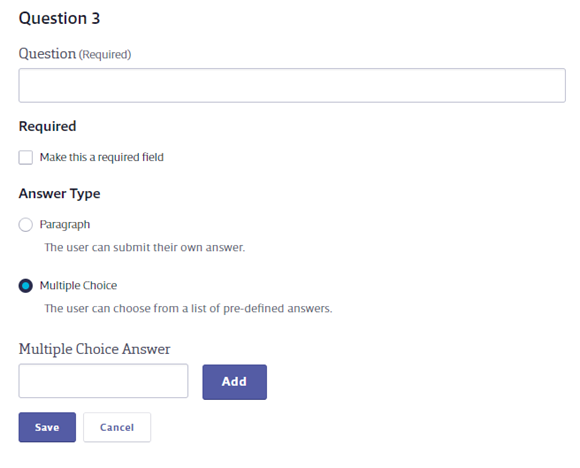
Enter the Question, determine whether or not it is required, and select the Answer Type. If you select Multiple Choice you will see answer fields. Enter the answer choice and click Add to create additional choices. Click Save below the Multiple Choice Answer field to save your question.
If you have staff using your Chemical Management system in languages other than English,
VelocityEHS recommends you use the Translate feature to ensure they see the correct wording. Click on Translate and then use Edit to enter the correct wording for the languages you need.
Once you’ve added all the submission questions, click Save to complete your Single-Step Approval workflow. Next, you’ll need to enable your workflow.
Creating a Multi-Step Approval Workflow
Your Chemical Management account comes with two types of Multi-Step Approval workflows:
- Independent Multi-Step Approval allows the Account Administrator to set up a chemical approval process whereby several departments can have a say in whether a chemical should be approved for the organization or not. These steps can be completed in any order.
- Sequential Multi-Step Approval allows the Account Administrator to set up a chemical approval process whereby several departments can have a say in whether a chemical should be approved for the organization or not. These steps must be completed in order.

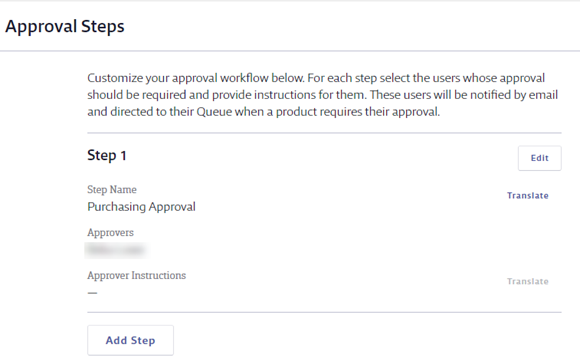
Once you’ve made your selection, the process of creating a Multi-Step Approval workflow is the same regardless of whether you choose Independent or Sequential. In both, you can create up to 5 approval steps.
To create an approval step, enter the Step Name and select the approver(s) for the step. Note that only Administrators or higher can be approvers for an Organization-Wide Approval workflow. If you need to include Managers as approvers, see Creating a Site-Specific Approval Workflow.
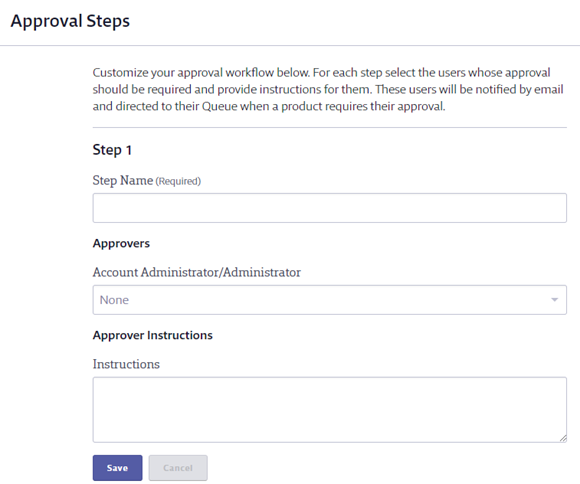
Enter any instructions for the approver and click Save.
If you have staff using your Chemical Management system in languages other than English,
VelocityEHS recommends you use the Translate feature to ensure they see the correct wording. Click on Translate and then use Edit to enter the correct wording for the languages you need.
To add additional steps, click Add Step. Repeat the process until you’ve created the steps you need.
Under Submitter Instructions, enter any instructions you need the person who is asking to add the safety data sheet to read. If you have staff using your Chemical Management system in languages other than English, VelocityEHS recommends you use the Translate feature to ensure they see the correct wording. Click on Translate and then use Edit to enter the correct wording for the languages you need.
If you have staff that do not have logins to your Chemical Management account but who need to be notified when a safety data sheet is approved or not approved, you can enter their emails in the Additional Notification Email section. You can enter multiple emails as long as they are separated by commas. The system will send the Product Approval Update Email to these addresses.
Use Attached Files if you have additional documents that need to be completed or reviewed by the submitter as part of the approval process. The submitter and any subsequent approvers can download the attached files and review them when submitting or approving the safety data sheet.
In Submission Questions create the questions you want the submitter to answer as part of asking to add the safety data sheet to your account. The Submission Questions are displayed to users whose safety data sheets require approval. Users that are Subject to Approval are directed to answer these questions after selecting a safety data sheet to add to the eBinder. See What does Subject to Approval mean? for more information.
Question 1 is always What is the reason for your submission? and cannot be edited or deleted. You can edit Question 2 by clicking on the Edit button. Question 2 can also be deleted.
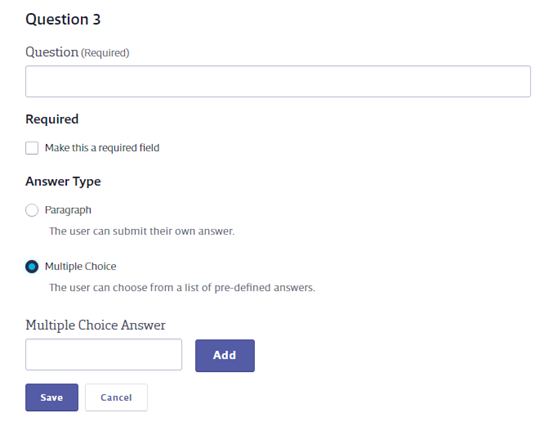
To add additional questions, click Add Question. You can have a total of 20 questions.
Enter the Question, determine whether or not it is required, and select the Answer Type. If you select Multiple Choice you will see answer fields. Enter the answer choice and click Add to create additional choices. Click Save below the Multiple Choice Answer field to save your question.
If you have staff using your Chemical Management system in languages other than English,
VelocityEHS recommends you use the Translate feature to ensure they see the correct wording. Click on Translate and then use Edit to enter the correct wording for the languages you need.
Once you’ve added all the submission questions, click Save to complete your Multi-Step Approval workflow.
Enabling Organization-Wide Approval Workflows
Once you’ve created your workflow using the steps above, you’ll need to enable it in your account. To enable a workflow you need to turn on Subject to Approval for the roles whose safety data sheet submissions need to go through the workflow steps.
For information about turning on Subject to Approval, see Configuring Queue and Approval Processes.
See Approving Safety Data Sheets in the Queue for information on adding safety data sheets to the eBinder as part of an approval workflow.


